To empower rare disease solutions through partnership, innovation, and scaled pre-clinical pipelines, to deliver targeted therapies from lab to clinic swiftly and effectively.

Partner with us to provide patients with rare disease and families an efficient plan from diagnosis to therapy.
Learn more about our collaborations

Are you passionate about rare disease research?Apply now and join our growing team.
![]()
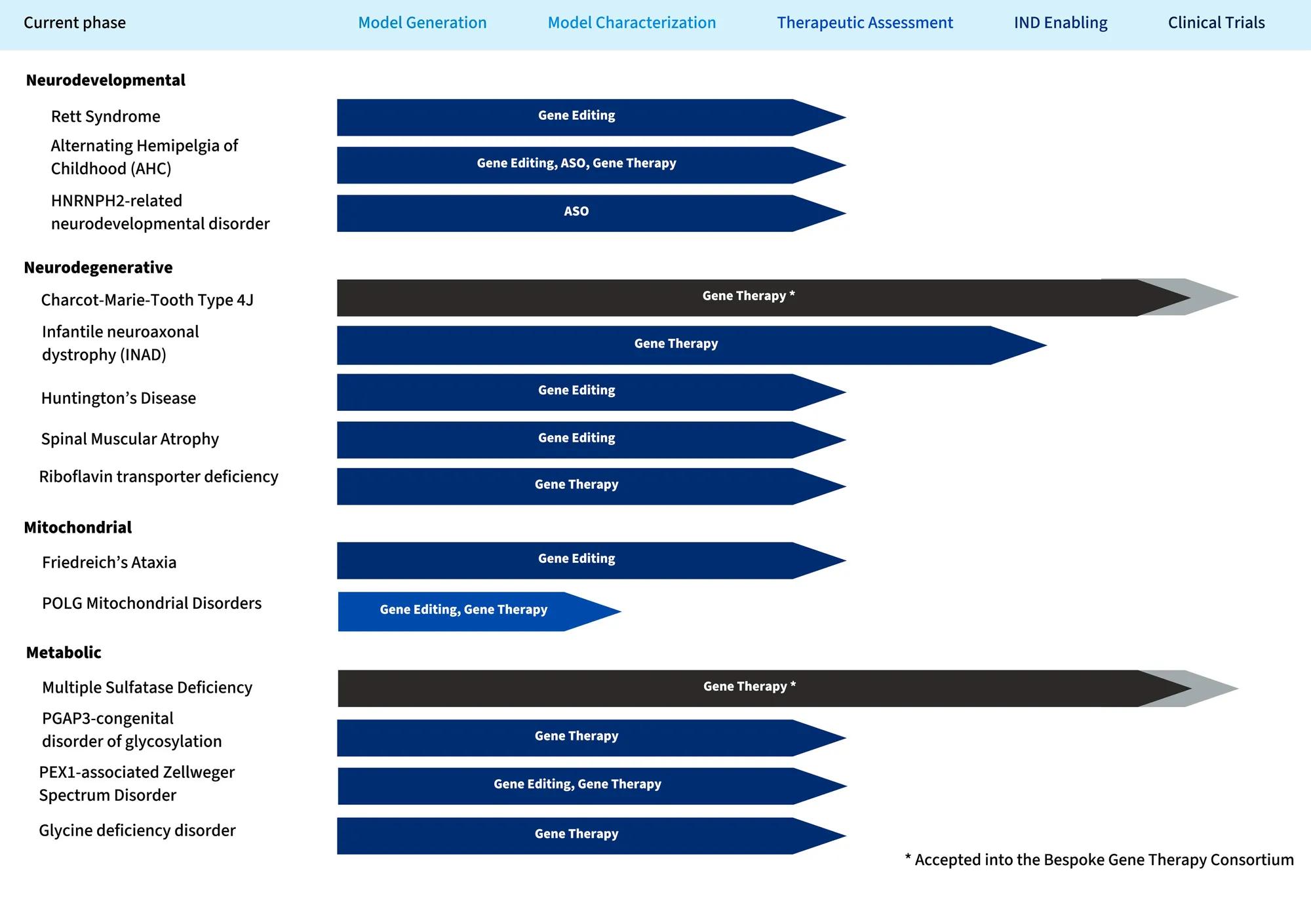
A mouse model is a foundational research platform to accelerate your preclinical research pipeline. Our team of scientists work closely with you to determine if a rare disease model exists and if it fits your therapeutic strategy. If no suitable model is available, our genetic engineers employ state-of-the-art technologies and strategies to effectively model patient variants. We aim to future-proof your model by designing it to be a tool to test a broad range of translational therapeutic approaches.
![]()
Our team of scientists work with you to craft preclinical pipelines with a focus on outcomes that translate directly to clinical practice. Our commitment to rigorous, robust, and reproducible research, positions your studies to be Investigational New Drug (IND) supporting. We are redefining the testing process to make high-quality preclinical work accessible and efficient by employing a parallel approach which, accelerate lead therapies to the clinic faster. This approach was the foundation for the successful clinical trials of Spinraza, the first FDA-approved drug for spinal muscular atrophy (SMA).
Design your preclinical platform
![]()
We collaborate with a diverse range of global entities, from families who have just received a diagnosis to biotech and pharmaceutical groups. As your partner, our research team not only executes your studies but also becomes an integral part of your scientific research team. We tenaciously tackle even the most complex research problems, drawing on decades of expertise in rare disease.
The JCPG prioritizes accessibility to these critical models in the otherwise resource-limited rare disease therapeutic research field. In collaboration with the Rare Disease Translational Center’s preclinical pipeline, the models incorporated into the program are used to test therapeutics, promoting their ultimate translation to the clinic. Learn more about our work at the JAX Center for Precision Genetics.

The Jackson Laboratory Center for Precision Genetics (JCPG) seeks to create and validate new, precise animal models of incurable and genetically complex human diseases. The JCPG was initiated in 2015 as one of three NIH-funded Pilot Centers.
View more